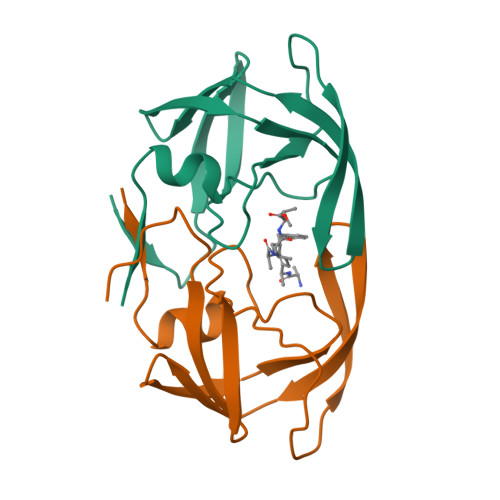
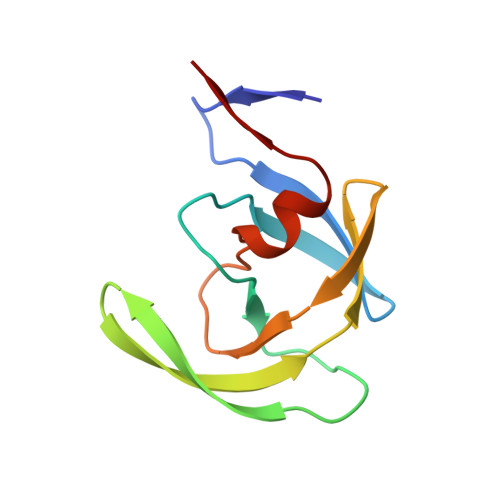
A check on rational drug design: crystal structure of a complex of human immunodeficiency virus type 1 protease with a novel gamma-turn mimetic inhibitor.
Hoog, S.S., Zhao, B., Winborne, E., Fisher, S., Green, D.W., DesJarlais, R.L., Newlander, K.A., Callahan, J.F., Moore, M.L., Huffman, W.F., Abdel-Meguid, S.S.(1995) J Med Chem 38: 3246-3252
- PubMed: 7650677
- DOI: https://doi.org/10.1021/jm00017a008
- Primary Citation of Related Structures:
1HBV - PubMed Abstract:
We have previously reported (Newlander et al., J. Med. Chem. 1993, 36, 2321-2331) the design of human immunodeficiency virus type 1 (HIV-1) protease inhibitors incorporating C7 mimetics that lock three amino acid residues of a peptide sequence into a gamma-turn. The design of one such compound, SB203238, was based on X-ray structures of reduced amide aspartyl protease inhibitors. It incorporates a gamma-turn mimetic in the P2-P1' position, where the carbonyl of the C7 ring is replaced with an sp3 methylene group yielding a constrained reduced amide. It shows competitive inhibition with Ki = 430 nM at pH 6.0. The three-dimensional structure of SB203238 bound to the active site of HIV-1 protease has been determined at 2.3 A resolution by X-ray diffraction and refined to a crystallographic R-factor (R = sigma magnitude of Fo magnitude of - magnitude of Fc magnitude of /sigma magnitude of Fo magnitude of, where Fo and Fc are the observed and calculated structure factor amplitudes, respectively) of 0.177. The inhibitor lies in an extended conformation in the active site; however, because of the constrained geometry of the C7 ring, it maintains fewer hydrogen bonds with the protein than in most other HIV-1 protease-inhibitor complexes. More importantly, the inhibitor binds to the enzyme differently than predicted in its design, by binding with the P2-P1' alpha-carbon atoms shifted by approximately one-half a residue toward the N-terminus from their presumed positions. This study illustrates the importance of structural information in an approach to rational drug design.
Organizational Affiliation:
Department of Macromolecular Sciences, SmithKline Beecham Pharmaceuticals, King of Prussia, Pennsylvania 19406, USA.